On December 20, 2024, Eli Lilly and Company announced that the U.S. Food and Drug Administration (FDA) has approved Zepbound® (tirzepatide) as the first and only prescription medication for adults with moderate-to-severe obstructive sleep apnea (OSA) and obesity. This groundbreaking approval offers hope to millions of individuals who suffer from these interconnected conditions. By tackling both sleep apnea and obesity, Zepbound presents a new avenue for effective treatment where previously limited options existed.
Understanding the Connection Between Sleep Apnea and Obesity
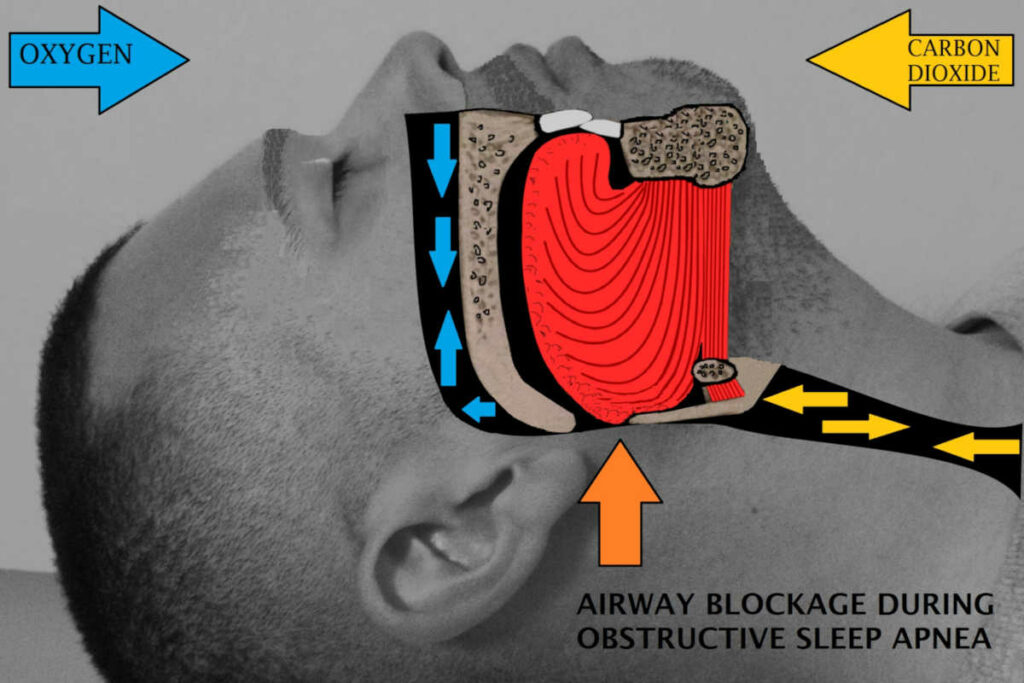
Sleep apnea is a sleep-related disorder characterized by repeated interruptions in breathing while a person sleeps. These interruptions, which can last for several seconds to minutes, occur when the airway becomes partially or fully blocked. While snoring is a common symptom, the condition can cause fatigue, excessive daytime sleepiness, and poor sleep quality, leading to a diminished quality of life. Obesity is one of the most significant risk factors. Excess fat around the neck and throat area can contribute to airway obstruction during sleep, which increases the likelihood of apneas and hypopneas (shallow breathing). As obesity rates continue to rise globally, the prevalence of sleep apnea has also increased.
Unfortunately, the condition is often undiagnosed or inadequately treated. Left unchecked, this condition can contribute to high blood pressure, heart disease, stroke, and even diabetes. The approval of Zepbound by the FDA is especially important as it offers a new solution for people who suffer from both obesity and sleep apnea, two conditions that are often treated separately.
Zepbound: A Breakthrough Treatment for Sleep Apnea and Obesity
Zepbound (tirzepatide) is a first-of-its-kind medication that combines two important therapeutic mechanisms. It activates both the glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors, which play critical roles in regulating appetite, glucose metabolism, and fat storage. As a result, Zepbound not only supports weight loss but also improves respiratory function, which is key in treating sleep apnea.
The FDA’s approval of Zepbound was based on the positive results from the SURMOUNT-OSA clinical trial. This large-scale, randomized study tested the effectiveness of Zepbound in adults with moderate-to-severe obstructive sleep apnea and obesity. The trial involved both patients who were using positive airway pressure (PAP) therapy and those who were not. Zepbound was administered in doses of 10 mg or 15 mg once a week.
Clinical Findings: Significant Improvement in Both Sleep Apnea and Obesity
The results from the SURMOUNT-OSA trial were striking. The study demonstrated that Zepbound was significantly more effective than a placebo in reducing the number of breathing interruptions during sleep. For participants not using PAP therapy, those on Zepbound experienced a reduction of 25 fewer breathing disruptions per hour, compared to just five fewer disruptions per hour in the placebo group. For those already using PAP therapy, Zepbound resulted in 29 fewer disruptions per hour compared to six with a placebo.
Furthermore, the trial showed substantial weight loss for participants on Zepbound. On average, adults lost 45 pounds (18%) over the course of a year. For those using PAP therapy in combination with Zepbound, the average weight loss was 50 pounds (20%). This stands in stark contrast to the placebo group, where participants lost only a few pounds.
These results indicate that Zepbound not only improves sleep apnea symptoms but also supports significant weight loss, making it an ideal option for individuals with both conditions. The weight loss associated with Zepbound can help alleviate the underlying causes of sleep apnea, particularly in individuals whose obesity contributes to their condition.
The Impact of Zepbound on Sleep Apnea Remission
Another critical finding from the clinical trial was the percentage of participants who achieved remission or mild non-symptomatic sleep apnea after one year of treatment. For those using Zepbound without PAP therapy, 42% saw their sleep apnea symptoms improve to the point where they experienced little to no disruption in their sleep. This number increased to 50% for individuals using Zepbound in combination with PAP therapy. In contrast, only 16% and 14% of placebo participants saw similar improvements, respectively.
This represents a major breakthrough in the management of moderate-to-severe obstructive sleep apnea, which has traditionally required mechanical treatments like CPAP machines or surgical interventions. By providing a pharmaceutical option that improves both sleep apnea symptoms and supports long-term weight loss, Zepbound could change the way healthcare providers treat this complex, chronic condition.
Why Zepbound is a Game-Changer for Obesity and Sleep Apnea
Zepbound’s approval is a significant moment in the fight against obesity and sleep apnea. For years, these two conditions have been treated separately, but they are deeply interconnected. Obesity is the leading cause of obstructive sleep apnea, and managing both conditions together can significantly improve patients’ overall health outcomes. Zepbound offers an effective, non-invasive solution that addresses both issues simultaneously.
The medication’s ability to reduce appetite and promote weight loss is crucial for individuals whose obesity exacerbates their sleep apnea. By addressing the root cause of airway obstruction, Zepbound helps improve breathing during sleep and supports more restorative, uninterrupted sleep. The fact that Zepbound is injectable and administered once a week offers added convenience for patients compared to daily pills or more invasive treatments.
In addition to improving sleep and promoting weight loss, Zepbound also has the potential to reduce the need for positive airway pressure (PAP) therapy in many patients. PAP therapy is considered the gold standard for treating sleep apnea, but it is not always well-tolerated. Patients who are unable to use PAP therapy due to discomfort or other reasons may find Zepbound to be an effective alternative.
Safety Considerations and Side Effects
As with any medication, Zepbound comes with certain risks and potential side effects. The most commonly reported side effects include nausea, diarrhea, vomiting, and constipation. These side effects were primarily mild and transient in the clinical trials, with most patients able to tolerate the treatment after an initial adjustment period.
However, Zepbound may cause more serious side effects, including thyroid tumors, which have been observed in animal studies. Kidney problems and gastrointestinal issues, such as pancreatitis and severe stomach pain, have also been reported. It’s crucial for patients to discuss their medical history with their healthcare provider before starting Zepbound, especially if they have any history of thyroid conditions, kidney disease, or severe gastrointestinal disorders.
A New Era in Sleep Apnea and Obesity Treatment
The FDA’s approval of Zepbound is a groundbreaking development in the treatment of moderate-to-severe obstructive sleep apnea and obesity. With its dual-action mechanism, Zepbound offers a comprehensive approach to treating these two chronic conditions that often go hand-in-hand. By improving both sleep quality and promoting weight loss, Zepbound presents a novel option for individuals who have struggled to manage these conditions separately.
For millions of people with sleep apnea and obesity, Zepbound’s approval offers new hope for a better quality of life. With continued research and broader access, Zepbound could become a standard treatment option, offering lasting health improvements for those affected by these two debilitating conditions.
Conclusion
Zepbound (tirzepatide) represents a major advancement in the treatment of sleep apnea and obesity. Its ability to reduce breathing interruptions during sleep while promoting significant weight loss makes it a unique and effective option for people living with both conditions. As more patients are diagnosed with sleep apnea and obesity, Zepbound’s approval could help transform the approach to managing these intertwined health challenges.
For more information on Zepbound and its treatment benefits, visit Zepbound.lilly.com.